We have investigated solid nitrogen as a system of linear molecules on a lattice. Solid N2 has been a system of interest for many years, and is used as an archetype for determining general properties of classical linear molecules. While much of this study has been done in parallel with hydrogen studies, hydrogen must be treated quantum mechanically, while nitrogen can be treated classically for all but the highest pressure phase [6]. Other classical linear molecules include O2, CO, and CO2.
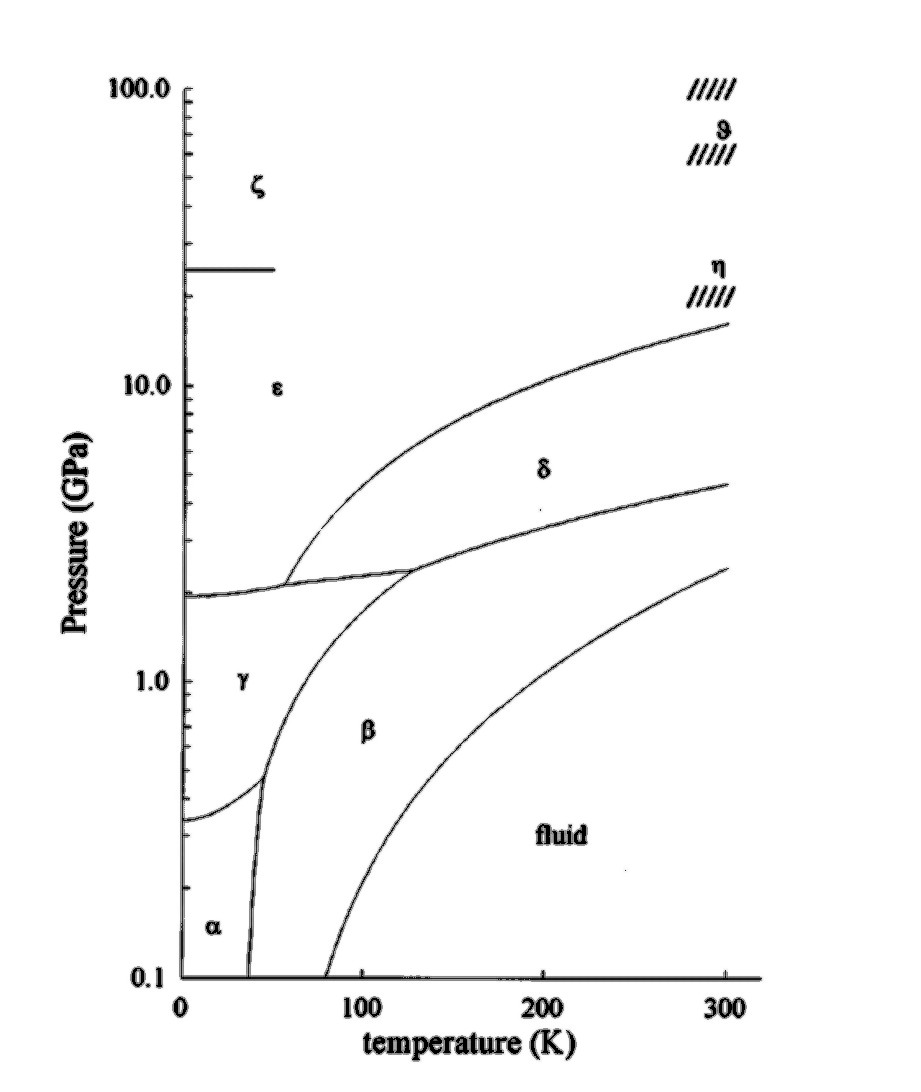
For our study we have chosen to study nitrogen on a lattice, using an orientational Metropolis Monte Carlo system. The molecules have been fixed to the lattice structures known from experiment. The phase diagram below (fig 1) shows the six different solid phases, alpha, beta, gamma, delta, epsilon and zeta. We have chosen to simulate the alpha, beta, and gamma phases, and have attempted to find phase transitions between the alpha and beta, alpha and gamma, and beta and gamma phases.
As our results show, although we did get reasonable results for our
potential energies for individual lattice structures, we were unable to
show the transitions.
 |
| Fig 1. The phase diagram of solid nitrogen, showing the six known solid phases. |
Next: The Lattices
Up to Table of Contents